
COVID-19 Virus Testing: The Current Outlook
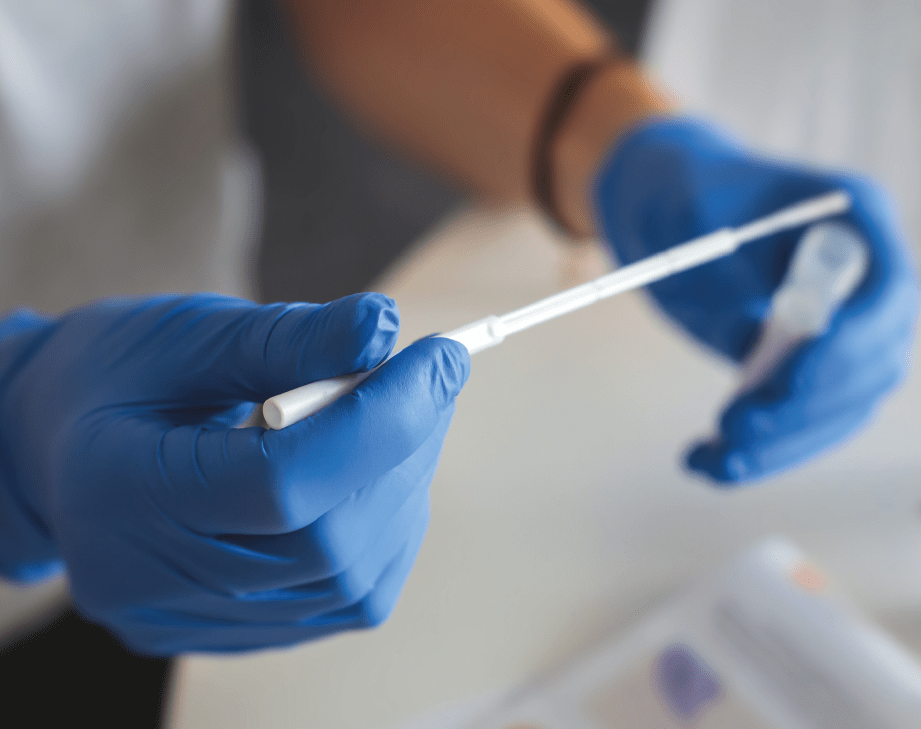
A COVID-19 virus test will identify whether somebody has COVID-19 at the time of taking a sample. It is also known as acute antigen testing or polymerase chain reaction (PCR) testing, which usually requires nasal and oral swabs.
The test itself is complex as it involves sampling viral RNA (Ribonucleic acid), and, using a reverse transcriptase process, it turns this into DNA (Deoxyribonucleic acid).
Using repeated amplification, it then produces a detectable result. This method produces a 70% positive test rate, as it is highly dependent on the quality of the sampling.
Current virus tests, or antigen tests - to see if you have COVID-19 now.
We've seen many businesses take proactive steps both to maintain business activities, and to prevent further transmission use this option. This is the current gold standard despite the limitations described above. A swab sample is taken by a clinician or trained technician, or on a self-administered basis (which is thought to have a lower sampling accuracy).
The tests we administer all carry a CE mark or are MHRA approved. These are processed by a major accredited laboratory, which integrates into our case management systems, and all positive tests are fed back into UK Government Track and Trace programme.
We are seeing employers use the COVID-19 antigen tests for a range of situations, including:
- To reassure employees and customers that there is no current COVID-19 infection in the workplace
- Proactive testing of a select population to maintain business continuity and operations
- To facilitate travel for internationally mobile populations
- To support with planning activities or events with higher risk of transmission
As this test must be sent to and processed by a laboratory, we aim to return test results within 48 - 72 hours of a swab being taken.
The downside to this test is it does not factor in the potential for an individual to be infected within the time the swab is taken to getting the results, unless individuals are in a controlled COVID-19 free environment.
Clearly there are rapid developments within the diagnostics industry, and it is likely that new, faster validated tests will become available in the coming weeks and months. Naturally, we are keeping this situation under continual review. We will not recommend any test that has not met full regulatory approval.



